Gallium Maltolate in the Treatment of Cancer: Summary
Lawrence R. Bernstein, PhD
Gallixa LLC • Menlo Park, California
Highlights
- Gallium maltolate (GaM) is under development as a drug for easy, once-per-day oral administration
- Oral GaM appears to be a safer and more convenient form of an FDA-approved drug (intravenous gallium nitrate, which must be administered continuously over five days in a hospital)
- Oral GaM has been extensively studied in several FDA-approved Phase 1 clinical trials, on more than 120 healthy volunteers and late-stage cancer patients
- Phase 1 clinical trials of oral GaM found the compound to have low toxicity and be well tolerated
- Apparent clinical efficacy of oral GaM, sometimes remarkable, has been reported in many advanced cancers, including lymphoma, liver cancer, breast cancer, lung cancer, colorectal cancer, bladder cancer, and prostate cancer
- GaM works against cancer by a novel mechanism. In the body, gallium behaves similarly to the form of iron essential for cancer cell multiplication, so it is avidly taken up by cancer cells, while being largely ignored by healthy cells. Once within the cancer cells, gallium acts as a "Trojan Horse", preventing the cells from reproducing, leading to their death
- Unlike most drugs, gallium from oral GaM crosses the blood-brain barrier, allowing it to reach brain cancers
- Oral GaM is a component of personalized ("precision") medicine: it can be paired with an FDA-approved diagnostic method (gallium-67 scan)
Overview
Today, millions of cancer patients receive treatments that cause severe side effects, and yet do little to prolong survival. Gallium maltolate (GaM) is a new, orally administered compound that has the potential to fundamentally improve the lives of many of these patients, by providing effective treatment without serious side effects. GaM is being developed as an orally active formulation of gallium, a drug already approved by the FDA in intravenous form (gallium nitrate for injection). Clinical and preclinical studies have repeatedly shown gallium to have efficacy against cancer, inflammatory disorders, infectious disease, and bone loss. Gallium is a particularly exciting new therapeutic because it naturally targets diseased tissue while sparing healthy tissues.
Chemistry
Gallium Maltolate
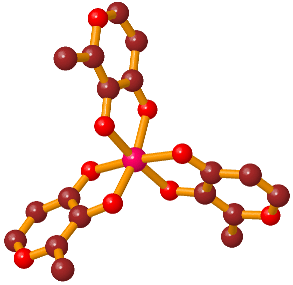
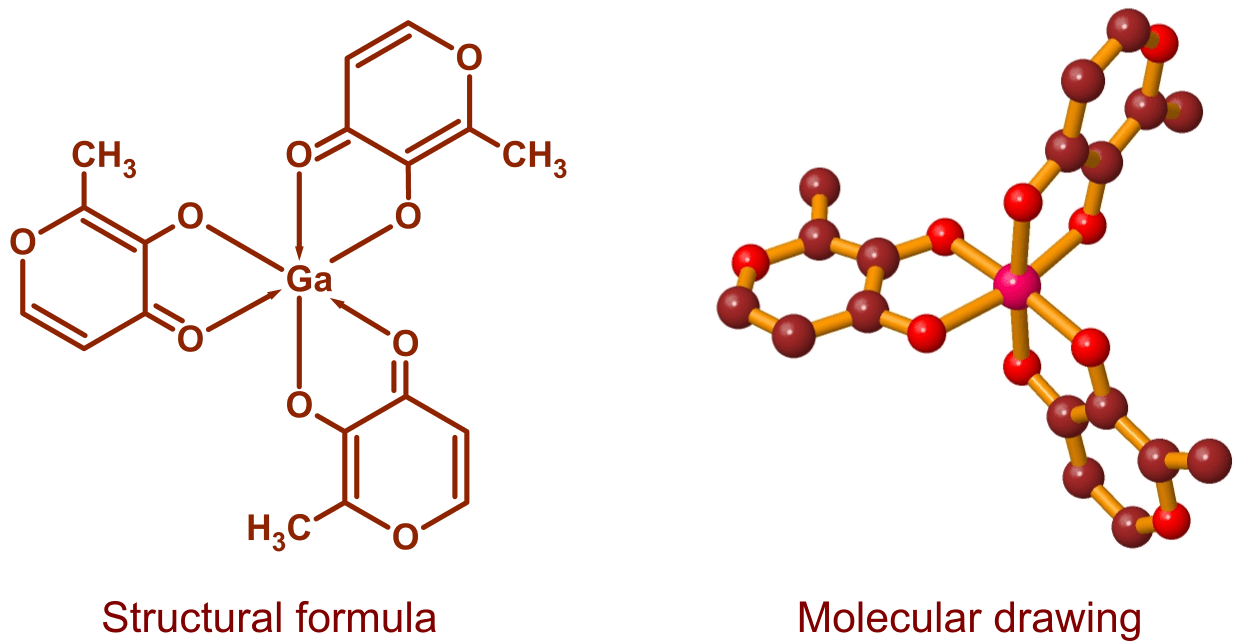
Gallium maltolate, tris(3-hydroxy-2-methyl-4-pyronato)gallium, is a coordination complex consisting of a gallium atom surrounded by three maltolate ligands. Maltol is a naturally occurring compound that is commonly produced during the cooking of foods containing sugars: it is responsible for the aroma of cotton candy and contributes to the fragrance of cookies, cakes, and other baked goods. Maltol is also found in some fruits, and is a widely used, FDA-approved food additive. Gallium maltolate is electrically neutral and moderately soluble in both water and lipids. It is stable over a pH range of approximately 5 through 8, a relatively large interval for a metal-organic complex.
Mechanism of Action
Chemically, gallium behaves remarkably like iron in its highly oxidized (trivalent) form (Fe3+; ferric iron). Unlike iron, however, which readily cycles between the ferric form and the less oxidized ferrous (divalent) form (Fe2+), gallium is always trivalent (Ga3+) under physiologic conditions. This means that gallium competes with ferric iron, which is required for the activity of some enzymes, but if it enters those enzymes, they are not able to function. It also means that gallium is not incorporated into hemoglobin or other vital molecules that contain ferrous iron (Fe2+); this lack of incorporation contributes to gallium's low toxicity.
Gallium administered orally as gallium maltolate appears to follow the normal uptake pathway used by iron. Absorption is primarily in the first part of the small intestine, where gallium separates from the maltolate ligand and becomes bound to transferrin in the blood plasma. Transferrin, the primary transport protein for iron, has two iron-binding sites per molecule; these binding sites are also able to accommodate gallium ions. Only about a third of the binding sites are typically occupied by iron, so sites are readily available for gallium.
Multiplying cancer cells have a high need for iron: in many cases, iron appears to be the limiting nutrient for cell division. The requirement for iron is due mostly to ferric iron’s position at the active site of ribonucleotide reductase (RR), an enzyme essential for DNA synthesis. To acquire iron, multiplying cancer cells express on their surface a large amount of transferrin receptor, which binds to metal-saturated transferrin in the blood. The complex of metal-saturated transferrin and transferrin receptor is taken into the cell by endocytosis; the metal ions are then released, and the transferrin and transferrin receptor are transported out of the cell and recycled. If gallium is present on the transferrin instead of iron, it will act as a Trojan Horse, being taken into the cancer cells, where it competes with intracellular iron and prevents RR from becoming functional. The resulting inability of the cell to synthesize DNA will halt cell division and lead to apoptosis (programmed cell death).
A further anticancer mechanism relates to gallium’s ability to strongly inhibit bone mineral degradation. This activity is thought to significantly inhibit the metastasis of cancer to bone and the destruction of bone by tumors. Animal and in vitro data suggest that gallium can, in addition, actually stimulate the regrowth of healthy bone (anabolic activity).
The ability of gallium to selectively target cancer tissue, on which it exerts anticancer activity by a unique mechanism (competition with ferric iron), together with its ability to potently inhibit bone mineral degradation, make gallium a very attractive therapeutic agent.
Clinical Experience
Intravenous gallium nitrate was approved by the FDA in 1991 for the treatment of cancer-related hypercalcemia. Efficacy of intravenous gallium nitrate against several cancers, particularly lymphomas, multiple myeloma, metastatic prostate cancer, and urothelial carcinoma, has been observed. Administration of this compound is, however, limited due to its kidney toxicity. Intravenous gallium nitrate is eliminated predominately by the kidneys (approximately 49-94% is excreted in the urine in 24 hours), where it may transiently reach high concentrations. In contrast, only about 2% of gallium orally administered as gallium maltolate is eliminated in the urine in 72 hours, and no sign of kidney toxicity has been observed for this compound. Gallium from oral gallium maltolate is nearly all protein-bound (to transferrin) in the blood plasma, whereas a high proportion of gallium from intravenous gallium nitrate appears to be present as the free gallate ion, [Ga(OH)4]ˉ. Gallate, being a small charged molecule, is rapidly excreted by the kidneys. It thus appears that oral gallium maltolate, by delivering gallium so that it becomes bound almost entirely to plasma transferrin, should be more efficacious with lower toxicity on a per dosage basis than intravenous gallium nitrate.
Gallium maltolate has been administered to healthy volunteers and to some late-stage cancer patients in Phase I clinical trials. It has been given at doses as high as 3,500 mg/day for multiple 28-day cycles, with no observed dose-limiting toxicity or serious drug-related adverse effects. The compound has shown high oral bioavailability with an elimination half-life of approximately 17 to 21 hours. Anecdotal efficacy has been observed in patients with lymphoma, end-stage liver cancer (hepatocellular carcinoma), metastatic prostate cancer, metastatic colon cancer, metastatic lung cancer, metastatic breast cancer, bladder cancer, multiple myeloma, brain cancers, and chronic lymphocytic leukemia.
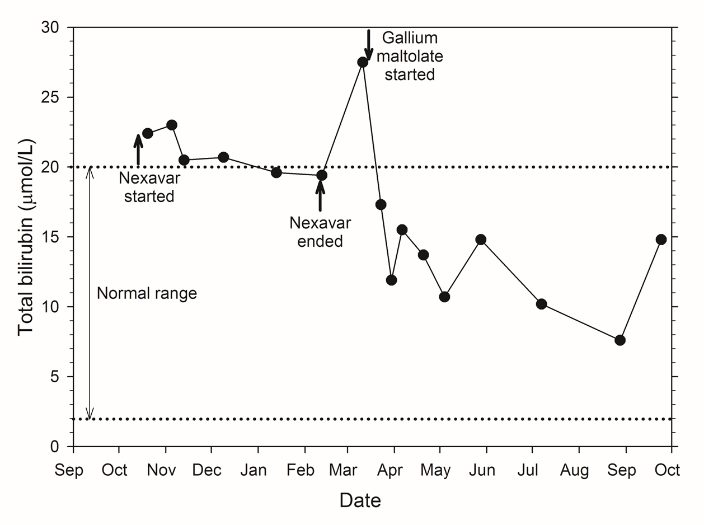
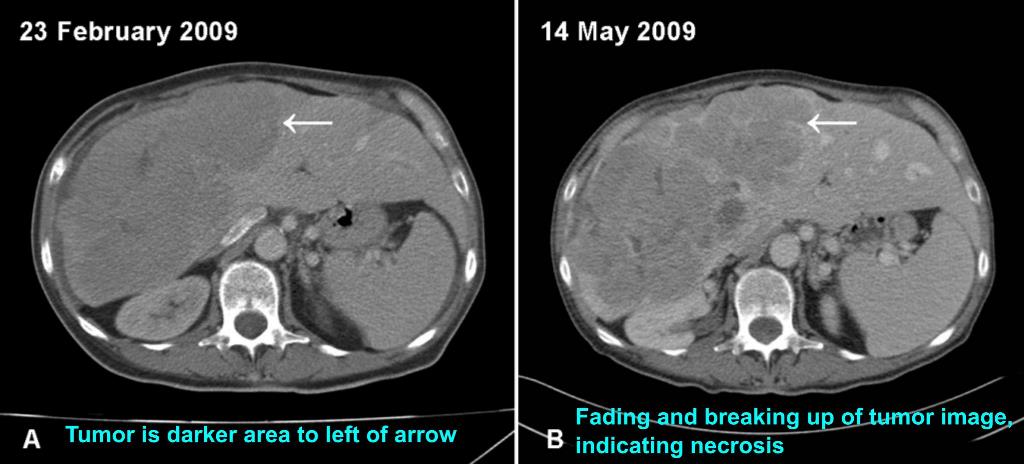
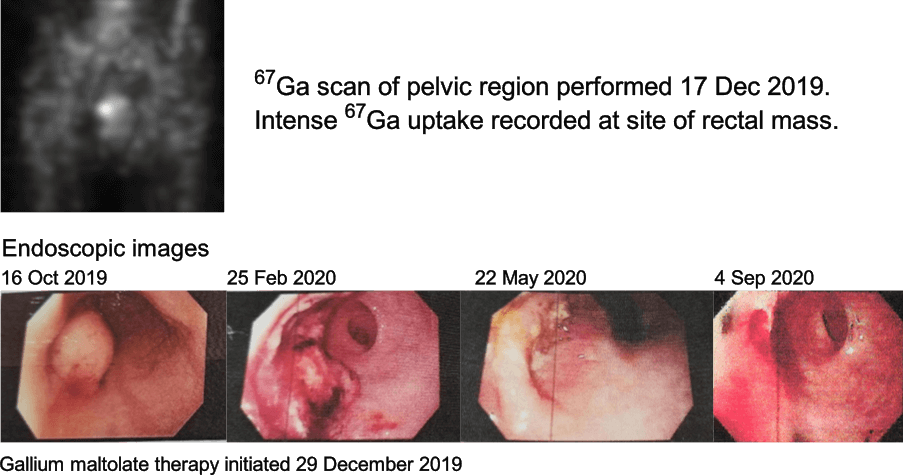
As an example, a patient with inoperable, late-stage liver cancer, who had not responded to chemotherapy with sorafenib (Nexavar®), was administered a daily dose of oral gallium maltolate. Prior to gallium maltolate treatment, the patient had severe right abdominal pain, elevated serum bilirubin, anorexia, and could not perform most routine activities. A gallium-67 scan showed avid gallium uptake by the 20 cm tumor. Within two weeks following the start of gallium maltolate treatment, the patient's right abdominal pain had decreased substantially. Two months following the start of treatment, the patient had resumed most normal activities, serum bilirubin was well within the normal range, and a CT scan showed apparent extensive necrosis and shrinkage of the tumor (see figures below).
In another example (see figures below), a 59-year-old woman had inoperable rectal adenocarcinoma. The main mass was approximately 3 cm in diameter at diagnosis, on 16 October 2019. The mass steadily decreased in size during the time the patient was on gallium maltolate monotherapy. No cancer was observed, either endoscopically or on biopsies, after eight months of the therapy.
Precision Medicine
Pharmaceutical therapy is increasingly trending towards a more personalized treatment of each patient. This trend is due to the recognition that different individuals, as well as different cases of a particular disease, may respond differently to the same drug. The use of gallium maltolate fits very well into this practice of precision medicine. Treatment with gallium maltolate can be targeted to those patients whose cancers are found to preferentially take up gallium in a gallium-67 scan: these are the patients most likely to respond to gallium maltolate therapy.
Selected Scientific Publications Regarding Gallium Maltolate
Selected Review Articles on the Therapeutic Applications of Gallium
Bernstein LR (2013) Gallium, therapeutic effects, in Encyclopedia of Metalloproteins, Kretsinger RH, Uversky VN, and Permyakov EA, eds., pp. 823-835, Springer, New York.Bernstein LR (2005) Therapeutic gallium compounds, in Metallotherapeutic Drugs and Metal-Based Diagnostic Agents: The Use of Metals in Medicine, Gielen M and Tiekink ERT, eds., pp. 259-277, Wiley, New York.
Bernstein LR (1998) Mechanisms of therapeutic activity for gallium. Pharmacological Reviews 50:665-682.
Anticancer Activity of Gallium Maltolate
Al-Gizawiy M, Wujek R, Zoss CJ, Tanaka R, Mirza SP, Chitambar C, Schmainda K (2023) HGG-06. Characterization of the effect of the oral iron-mimetic gallium maltolate on pediatric glioblastoma growth in vivo. Neuro-Oncology 25(Suppl 1):i39, DOI: 10.1093/neuonc/noad073.155Al-Gizawiy M, Wujek R, Zoss CJ, Tanaka R, Mirza SP, Chitambar C, Schmainda K (2023) ATRT-03. The oral iron-mimetic gallium maltolate inhibits ATRT in vivo – imaging and histological characterization. Neuro-Oncology 25(Suppl 1):i1, DOI: 10.1093/neuonc/noad073.003
Al-Gizawiy M, Wujek R, Prah M, Connelly J, Chitambar C, Schmainda K (2022) EXTH-16. The oral iron-mimetic gallium maltolate supresses treatment-resistant glioblastoma – in vivo validation. Neuro-Oncology 24(Suppl 7):vii212, DOI: 10.1093/neuonc/noac209.815.
Molino S, Chitambar CR (2020) Involvement of the lysosome in the cytotoxicity of gallium maltolate in human pediatric brain tumor cell lines [abstract]. In: Proceedings of the Annual Meeting of the American Association for Cancer Research 2020, Apr 27-28 and Jun 22-24, 2020, Philadelphia (PA); Cancer Research 80(16 Suppl):Abstract nr 4176.
Alhajala HS, Markley JL, Kim JH, Al-Gizawiy MM, Schmainda KM, Kuo JS, Chitambar CR (2020) The cytotoxicity of gallium maltolate in glioblastoma cells is enhanced by metformin through combined action on mitochondrial complex 1. Oncotarget 11:1531-1544.
Molino S, Al-Gizawiy MM, Knipstein J, Schmainda KM, Chitambar CR (2019) Gallium Maltolate as Treatment for Pediatric Glioma. 5th Pediatric Neuro-Oncology Basic and Translational Research Conference, San Francisco, CA, May 3-4, 2019, Poster HGG-05.
Chitambar CR, Al-Gizawiy MM, Alhajala HS, Pechman KR, Wereley JP, Wujek R, Clark PA, Kuo JS, Antholine WE, Schmainda KM (2018) Gallium maltolate disrupts tumor iron metabolism and retards the growth of glioblastoma by inhibiting mitochondrial function and ribonucleotide reductase. Molecular Cancer Therapeutics DOI: 10.1158/1535-7163.MCT-17-1009.
Al-Gizawiy M, Robert W, Alhajala H, Cobb J, Prah M, Mirza S, Chitambar C, Schmainda K (2018) Oral gallium maltolate impairs tumor growth and extends disease-specific survival in a xenograft model of recurrent GBM. Neuro-Oncology 20(Suppl 6):vi95.
Wu X, Wang TW, Lessmann GM, Saleh J, Liu X, Chitambar CR, Hwang ST (2015) Gallium maltolate inhibits human cutaneous T-cell lymphoma tumor development in mice. Journal of Investigative Dermatology 135:877-884.
Bernstein LR, van der Hoeven JJM, Boer RO (2011) Hepatocellular carcinoma detection by gallium scan and subsequent treatment by gallium maltolate: rationale and case study. Anti-Cancer Agents in Medicinal Chemistry 11:585-590.
Chitambar CR, Purpi DP (2010) A novel gallium compound synergistically enhances bortezomib-induced apoptosis in mantle cell lymphoma cells. Leukemia Research 34:950-953.
Chitambar CR, Purpi DP, Woodliff J, Yang M, Wereley JP (2007) Development of gallium compounds for treatment of lymphoma: gallium maltolate, a novel hydroxypyrone gallium compound, induces apoptosis and circumvents lymphoma cell resistance to gallium nitrate. The Journal of Pharmacology and Experimental Therapeutics 322:1228-1236.
Chua MS, Bernstein LR, Li R, So SKS (2006) Gallium maltolate is a promising chemotherapeutic agent for the treatment of hepatocellular carcinoma. Anticancer Research 26:1739-1744.
General Scientific Articles on Gallium Maltolate
Enyedy ÉA, Dömötör O, Bali K, Hetényi A, Tuccinardi T, Keppler BK (2015) Interaction of the anticancer gallium(III) complexes of 8-hydroxyquinoline and maltol with human serum proteins. Journal of Biological Inorganic Chemistry 20:77-88.Ball KR, Sampieri F, Chirino M, Hamilton DL, Blyth RI, Sham TK, Dowling PM, Thompson J (2013) Synchrotron X-ray fluorescence microscopy of gallium in bladder tissue following gallium maltolate administration during urinary tract infection. Antimicrobial Agents and Chemotherapy 57:5197-5201.
Bernstein LR, Tanner T, Godfrey C, Noll B (2000) Chemistry and pharmacokinetics of gallium maltolate, a compound with high oral gallium bioavailability. Metal Based Drugs 7:33-48.
Bernstein LR (1995) Powder x-ray crystallography of gallium 3-hydroxy-4-pyronates. Powder Diffraction 10:140-142.
Additional references on gallium maltolate can be found at www.gallixa.com/GaMReferences.html
We are seeking partners, investors, or licensees to help complete the clinical trials needed to obtain FDA approval of gallium maltolate, which would make the compound available to those who may benefit from it. If you might be interested in joining us, or for further information, please contact: